SciTech Tuesday–Nuclei are sticky drops, not brittle solids, and that leads to The Bomb
For most of World War II, like in all of previous human history, people used energy stored in the bonds of molecules for power. But in December of 1938, a chain of events began that changed the way we use chemicals for energy—and human history changed drastically.
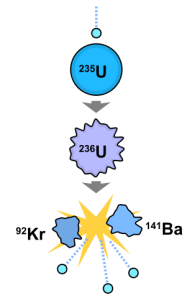
This diagram shows how a neutron entering the nucleus of uranium creates an unstable isotope, which breaks down into two other elements and releases protons and a great deal of energy.f
Chemistry and knowledge about the structure of atoms developed by leaps and bounds in the early 20th century. In 1869 Dimitri Mendeleev showed that all chemical elements can be grouped and organized using regular patterns in their characteristics. The Periodic Table of Elements was a huge innovation that allowed systematic investigation into the nature of atoms and elements. JJ Thomson’s discovery of the electron, and the modern atomic theory, began to reveal why those patterns in the Periodic Table existed.
The discovery that some elements had different forms with different masses (in 1913) led to the concept of isotopes (a term created by Margaret Todd) and a rush of investigation into the nucleus of atoms. From the work of Thomson on, much of the empirical work into the structure of atoms involved aiming electrons or radiation at them to see what would happen. This is how Ernest Rutherford discovered the atomic nucleus (1911), and the proton (1918). In 1932 Alex Chadwick described the neutron, and the full complement of atomic particles was available to theoreticians and chemists to explain quantum mechanics and chemical reactions.
Norman Feather bombarded nitrogen with neutrons, and found that the result was boron and alpha radiation. After this, neutrons were used to examine materials to look for heavier elements and radioactive elements. In the mid-1930s a research team led by Otto Hahn, Lise Meitner, and Fritz Strassman had found many new products when they exposed uranium to a beam of neutrons. Atomic theory had developed by thinking of all elements as more complicated forms of hydrogen, and as brittle material from which small parts might be chipped off. So scientists expected to break large atoms up by knocking off small parts when they shot them with neutrons. Lise Meitner had to flee to Sweden, since she was Jewish, and the lab was in Germany, but Hahn and Strassman continued the experiments and shared with her their data.

This was the setup used for the experiments by Hahn, Meitner, and Strassman, later replicated by Frisch. By today’s standards it’s very primitive.
In experiments with uranium exposed to a beam of electrons from December 16-17 1938, Hahn and Strassman found that the products were not forms of uranium, but were instead barium. On December 19th Meitner received a letter from Hahn describing the results. He was at a loss to explain how the atoms lost 40% of their mass. Meitner trusted that Hahn had identified the element correctly—after all he was an excellent analytical chemist. She understood the physics better than he did. Meitner also recalled that Marie Curie and her lab often found barium in samples of decayed radioactive material. She also knew that pioneers like Niels Bohr had described the nucleus as more like a liquid drop than a solid. Making some calculations, Meitner saw that the charged nucleus could in fact split into two large pieces. She saw also that the mass of the two resulting nuclei would be less than the mass of the original nucleus. Rather than being a problem, this difference in mass was more evidence that the nucleus had split. Einstein’s theory of relativity suggested that matter could be converted into energy. She made some more calculations and discovered that the missing matter could explain the huge energy that had been released in the transformation.
Lise Meitner worked with her visiting nephew Otto Frisch to write a detailed explanation of what they thought had happened in the experiment. When Frisch returned to his lab he reproduced the experiment and showed that they were correct in their interpretation. Meitner named the splitting of the nucleus ‘fission,’ after the splitting of cells in organisms. In 1944 Hahn, but not Meitner, was awarded the Nobel prize for the discovery of fission.
This new form of chemical manipulation—the splitting of nuclei instead of the splitting of molecules by breaking the covalent bonds between them—provided millions of times more energy. In just seven years this energy was used to make the world’s first three atomic bombs.
Posted by Rob Wallace, STEM Education Coordinator at The National WWII Museum




Leave a Reply