SciTech Tuesday: Glowing gin and tonics, bitter dyes, war propaganda
Have you noticed that your gin and tonic glows in the dark? Ever wondered why tonic water has that wonderfully bitter taste? Have you thought about how developing dyes led to medical advances? Curious what this has to do with World War II? Read on.
In 1940 one of the last remnants of the formerly powerful Dutch colonial system was the Kina Bureau, a cartel that monopolized quinine distillation. Quinine is an organic molecule that is extracted from the quina tree of South America. The Quecha people of the Andes cultivated the plant. They used it’s muscle relaxing properties to prevent shivering when they got cold. Jesuit missionaries noted that it also prevented malaria, and used it in their colonial missions. It was brought to Europe, called fever tree bark, in the early 17th century, and used in Spain and Italy where malaria was common. Peru and other countries began to try to control the seeds of the species of Cinchona that were medicinally useful shortly after that. However, merchants were successful at smuggling seeds and plant cuttings, and plantations were developed by the British in Sri Lanka, and by the Dutch in Indonesia in the 19th century. Scientists worked to synthesize the chemical in the lab throughout the 19th century, but were unsuccessful. Colonists were given a tincture of quinine in soda water to prevent malaria. Because the beverage had a bitter flavor there developed a habit of mixing it with something to mask the flavor—and thus the gin and tonic was born.
In the late 19th century and the turn of the 20th, German scientists were leaders in the use of coal tar to make synthetic dyes. Over the first 30 years of the 20th century they discovered that many of their dyes had medical uses (this was how sulpha drugs were discovered). The German industrial producer IG Farben synthesized a chemical they introduced as an alternative to quinine in the early 1930s. However, in addition to being less effective, this pill had terrible side effects including nausea.
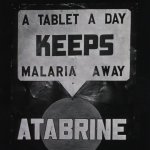
That was the context when the Japanese took control of southeast Asia, and the Germans took over the Netherlands. As tensions were mounting, the Secretary of Commerce failed to purchase large quantities of quinine, in spite of being instructed to do so, because he felt the price was too high. When the allies deployed soldiers to the Pacific, the results were serious. One year after Pearl Harbor, 8500 soldiers were hospitalized with malaria, and 50-80% of soldiers in field hospitals were there for malaria. Atabrine was being distributed, but its side effects and a Japanese propaganda campaign made soldiers unwilling to use it. You’ve seen those posters to promote malaria prevention for soldiers? They were the result of Japanese radio propaganda telling servicemen that atabrine caused impotence.
The government moved to find quinine in South and Central America, where the Axis powers didn’t have control over the Cinchona’s native forests. Agreements were made for access to the forests, and government scientists went in search of plants with high quantities of quinine in their bark. These plants would be used to develop breeding stock for new plantations. In the meantime these wartime botanists collected Cinchona bark for distillation of quinine. Braving poor conditions, disease (including malaria!), and other hardships, up to 40 scientists with native support scoured tropical forests at high altitudes. Eventually they sent to the U.S. 12.5 million lbs of bark, but they never found a high-yield strain. Hybridization and local conditions seemed to control production of quinine in the plants more than genetics. In 1944 the synthesis of quinine was successfully achieved by American scientists. The synthesis is still very complicated and inefficient, and so was never brought to commercial production. Although great advances have been made in malaria treatment in the last decades, treatments are still not as good as the international malaria problem requires—and the plasmodium that causes malaria seems adept at evolving resistance to treatment.
So fill up that glass with tonic water before you sit out in the summer evening. But fill it up often. Today the FDA limit on quinine in tonic water is 83mg/l. You’ll need to drink 10 liters a day to get your medicinal dose.
And one more thing…
Quinine is highly fluorescent in a mild acid solution. So much so that it is used as an international standard in analytical chemistry. Since carbonated water is mildly acidic, your tonic drink will glow in a black light. That black light is less likely to attract mosquitoes as well.
- A 19th Century illustration of a species of Cinchona. From Wikimedia Commons.
- From the US Army Medical Dept's Office of Medical History
- From the National Library of Medicine
This is the last post in a series on plant products in the war. Next week starts a run on the Manhattan Project and its scientists.
Posted by Rob Wallace, STEM Education Coordinator at The National WWII Museum.






Leave a Reply